CO2 is the most common source of general corrosion in oil and gas production. In the presence of water, CO2 forms carbonic acid which is corrosive. Water condensation often occurs in gas fields if the temperature falls below the dew point. This can particularly be a problem during start-up or shut-in. In addition to CO2 and water content, the corrosion rate depends upon the temperature, pH, flow rate, and presence of oxygen or organic acids. Acidizing and completion fluids may also contribute to the corrosive conditions.
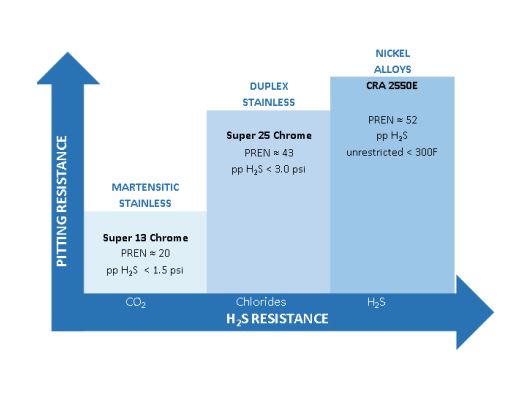
The corrosion-resistant alloys provide corrosion protection as a result of their chromium content. The chromium creates a protective layer. The higher the chromium content, the more effective the protective layer. Under mild conditions the martensitic stainless steels containing 13% chromium may be adequate, however, if the pH falls below 3.5, higher alloys such as the duplex stainless steels are recommended. 13% chromium steels should not be used for water injection wells without careful modeling of pH for both current and future well conditions.

*While every effort has been made to ensure the accuracy of the above review, assessment, conclusions, and report, the appropriateness of their application and their interpretation remain the sole responsibility of the user.
"*" indicates required fields